Spotlights
[Research News] Study Reveals Novel Therapeutic Target to Eliminate Unwanted and Misfolded Proteins
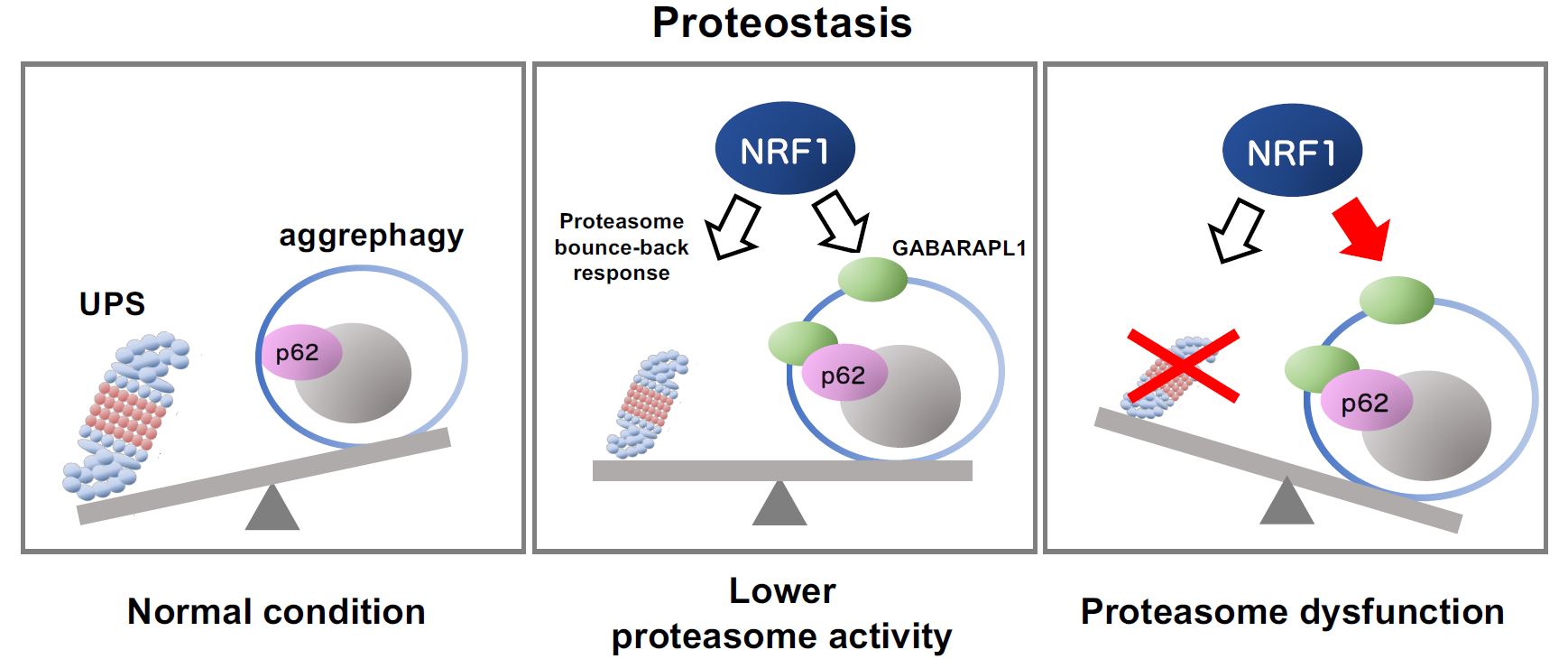 Schematic representation of NRF1-triggered activation of aggrephagy mediated by impaired proteasome activity
Schematic representation of NRF1-triggered activation of aggrephagy mediated by impaired proteasome activityThe ubiquitin‒proteasome system (UPS) and autophagy are protein degradation pathways essential for maintaining protein balance and regulation, or proteostasis (left panel). When proteasome activity decreases because of several reasons including chemical inhibitors and aging, the transcription factor NRF1 gets activated, leading to the upregulation of proteasome gene expression (“the proteasome bounce-back response;” middle panel). Furthermore, complete proteasome dysfunction activates NRF1-mediated aggrephagy, inducing the expression of the aggrephagy-related genes p62 and GABARAPL1 (right). These cellular responses help combat proteasome dysfunction by maintaining proteostasis.
Atsushi Hatanaka, a graduate student, and Akira Kobayashi, a professor, both from the Laboratory for Genetic Code, Graduate School of Life and Medical Sciences, and their research team discovered the hidden mechanism of "aggrephagy," with major implications for degenerative protein diseases.
In cells, the ubiquitin‒proteasome system (UPS) plays a key role in the elimination of unwanted or misfolded proteins. When UPS fails, cells activate a backup process called "aggrephagy" for clearing ubiquitin-tagged proteins. However, the associated mechanism behind this process remains unknown. Recently, Hatanaka, Kobayashi, and their colleagues have demonstrated how another protein called NRF1 facilitates aggrephagy, thereby providing new therapeutic targets for diseases resulting from misfolded proteins.
These findings pave the way toward developing novel therapeutics for degenerative diseases such as Alzheimer's disease, Parkinson's disease, and dementia with Lewy bodies.
Reference
Hatanaka A., Nakada S., Matsumoto G., Satoh K., Aketa I., Watanabe A., Hirakawa T., Tsujita T., Waku T., Kobayashi A. The transcription factor NRF1 (NFE2L1) activates aggrephagy by inducing p62 and GABARAPL1 after proteasome inhibition to maintain proteostasis (2023) Scientific Reports, 13 (1), art. no. 14405
DOI: 10.1038/s41598-023-41492-9
For more details, please see the website of Organization for Research Initiatives and Development, Doshisha University.
Research News: Study Reveals Novel Therapeutic Target to Eliminate Unwanted and Misfolded Proteins
This achievement has also been featured in the “EurekAlert!.”
NEWS RELEASE 4-OCT-2023 Study Reveals Novel Therapeutic Target to Eliminate Unwanted and Misfolded Proteins
Image Credit: Atsushi Hatanaka, Sota Nakada, Gen Matsumoto, Katsuya Satoh, Iori Aketa, Akira Watanabe, Tomoaki Hirakawa, Tadayuki Tsujita, Tsuyoshi Waku, and Akira Kobayashi
License type: CC BY 4.0
| Contact |
Department of Research Planning PHONE:+81-774-65-8256 |
|---|
Category
- 同志社大学公式サイト:
- Top Page /Research /International /Current Student /Alumni Student /