Spotlights
[Research News] Efficient CO₂ Conversion to Fuels and Chemicals Using Ionic Liquid Electrolyte
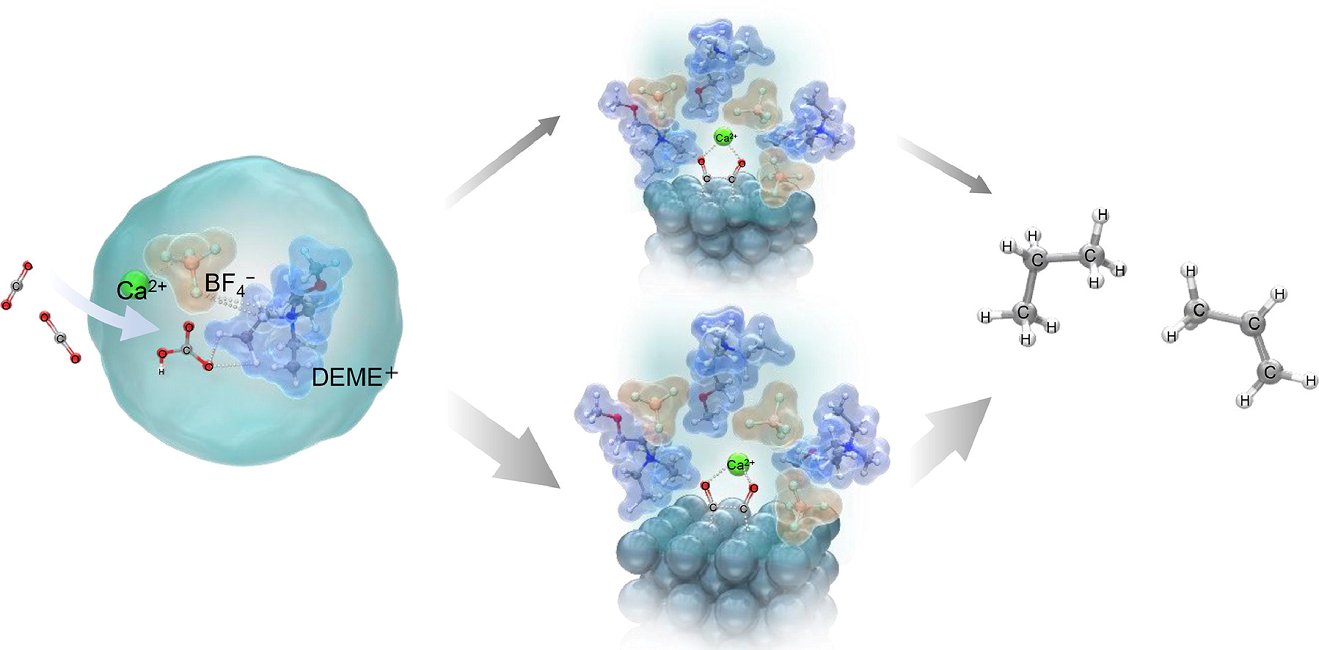 The CO₂ conversion process at the interface between DEME-BF₄ electrolytes containing Ca(OH)₂ aqueous solution and silver electrodes
The CO₂ conversion process at the interface between DEME-BF₄ electrolytes containing Ca(OH)₂ aqueous solution and silver electrodes The production of hydrocarbons occurs through two intermediates formed on the surface of the silver electrode to produce useful hydrocarbons like ethylene, ethane, propylene, and propane.
The research team, led by Professor Takuya Goto and including Ms. Saya Nozaki from the Graduate School of Science and Engineering and Dr. Yuta Suzuki from the Harris Science Research Institute, discovered that combining ionic liquids electrolytes with metal hydroxides enables efficient electrochemical conversion of CO₂ to hydrocarbons.
The electrochemical conversion of captured carbon dioxide into fuels and chemicals offers a sustainable approach to reduce emissions. However, traditional methods rely on complex electrode designs. Goto and his team demonstrated a cost-effective approach using an ionic liquid combined with metal hydroxides as an electrolyte, enabling efficient conversion even on basic metal electrodes. With this electrolyte, they successfully produced propane and ethylene directly from CO₂ and H₂O, offering a cost-effective method for emissions reduction.
Reference
Saya Nozaki, Yuta Suzuki, Takuya Goto, Electrochemical synthesis of C₂ and C₃ hydrocarbons from CO₂ on an Ag electrode in DEME-BF₄ containing H₂O and metal hydroxides, Electrochimica Acta, Volume 493, 2024,144431.
https://doi.org/10.1016/j.electacta.2024.144431
For more details, please see the website of Organization for Research Initiatives and Development, Doshisha University.
Research News: Efficient CO₂ Conversion to Fuels and Chemicals Using Ionic Liquid Electrolyte
This achievement has also been featured in the “EurekAlert!.”
NEWS RELEASE 3-JUNE-2024, Efficient CO2 Conversion to Fuels and Chemicals Using Ionic Liquid Electrolyte
Image Credit:Takuya Goto from Doshisha University
License type: CC BY 4.0 DEED
| Contact |
Department of Research Planning PHONE:+81-774-65-8256 |
|---|
Category
- Doshisha University Official Website:
- Top Page /Research /International /Current Student /Alumni Student /